Wound Closure Device
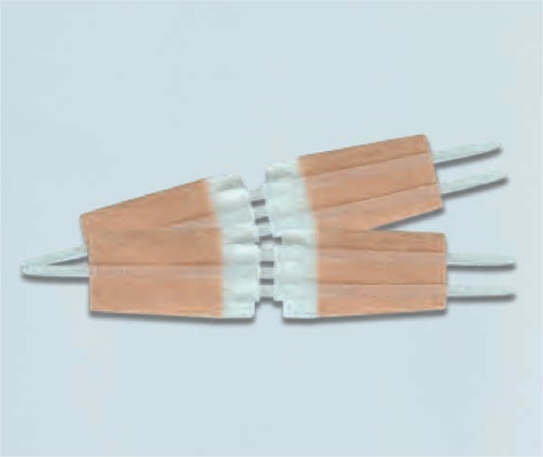
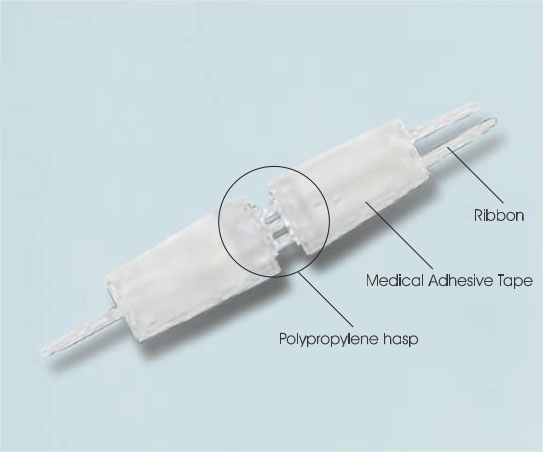
Wound closure device information
Wound closure device consists of a pair of polypropylene hasps with two adhesive panels.
The adhesive panels are porous nonwovens coated with a pressure-sensitive hypoallergenic adhesive.
It is a novel device for the quick and easy closure of most acute wounds, for example lacerated and surgical wounds.
The device is designed to allow for a non-invasive closure of a wound and to encourage a tight closure of the wound for an early and successful healing. The non-invasive technique reduces tissue trauma and improves patient comfort, and possibly causes less scarring after healing.
Features
Wound closure device is indicated for the closure
of acute wounds such as lacerations and surgical
incisions. It may also be used in conjunction with
subcutaneous suture operations.
- Patented technology
- Non-invasive closure, no sutures, less scar
- Quick to apply
- Easy to use
APPLICATION
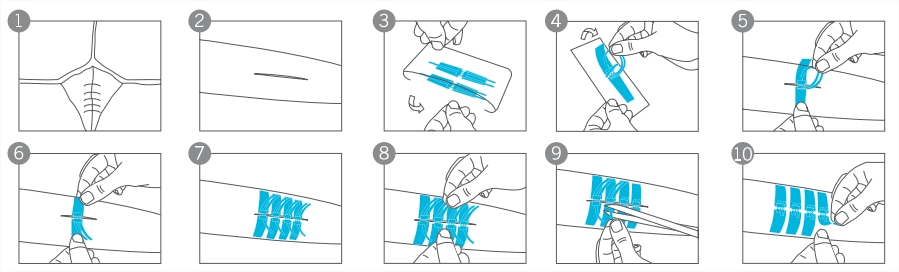
1. For patients with deep wounds, subcutaneous suture operation can be performed according to local clinical practice. See Figure 1.
2. Clean and dry the skin around the wound, making the wound edges as neat as possible.
See Figure 2.
3. Take the wound closure device out of the package, peel the hasp off the liner by lifting it from the ribbon ends.
See Figure 3 and 4.
4. Position the device so that the two adhesive panels sit on the two sides of the wound.
See Figure 5.
5. Press the adhesive panel with your finger gently along the direction of the ribbons so that the adhesive panel adheres firmly on the skin.
See Figure 6.
6. Apply the next hasp in the same way so that the gap between the hasps is about 2mm. See Figure 7.
7. Use both hands to hold the ribbons of the hasp and to tighten the hasp gently until the hasps are locked to close the wound. See Figure 8.
8. Cut off all long ends from the ribbons. See Figure 9.
9. Apply a PU film dressing or another secondary dressing to protect the hasp and wound. See Figure 10.
10. The secondary dressing can be removed for observation. A new secondary dressing must be applied after observation.
11. When the wound is healed, remove the hasp by lifting either the side of the adhesive panel and peel from the wound gently. Care must be taken to avoid damaging of the newly healed wound.
Frequently asked questions about Drainage Catheter
The product codes in this chart are for CVC kits with straight introducer needle configuration.
CVC kits with Y-shaped introducer needle configuration are also available in adult types. The product code of CVC kit with Y-shaped introducer needle configuration is to add letter “Y” after the product code of CVC kit with straight introducer needle configuration, for example, “FC-2726Y”.
Before you order, please clarify which configuration you request, either straight or Y-shape introducer needle.
Pediatric types of CVC include single lumen 22Ga, 20Ga, 18Ga; double lumen 4Fr, 5Fr;triple lumen 4.5Fr and triple lumen 5.5Fr.
Product customization on demand, don’t hesitate to contact us for more information.
